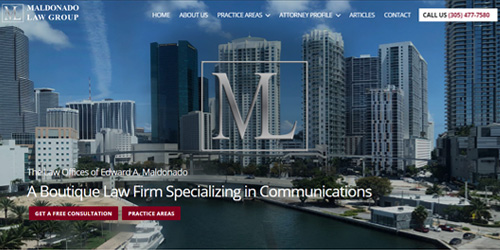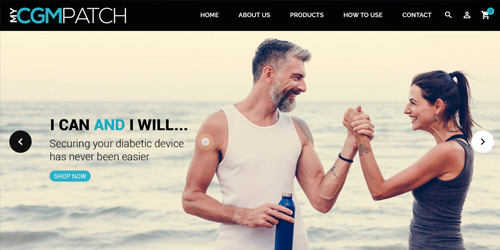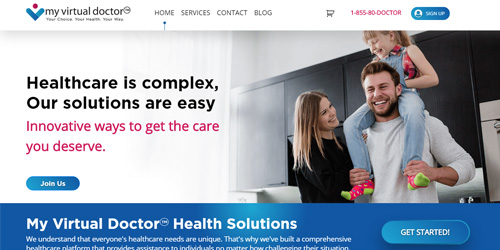
Crafting DigitalExperiences
Leveraging cutting edge technologies, from Mobile to Machine Learning, to craft innovative Digital Experiences
UI
Combining classical design principles with rapidly evolving digital media to create future-ready user interfaces
UX
Intermingling of creativity and technology to reengineer user experience for better end user engagement and outcomes
Development
Leverage the benefits of Continuous Integration and Deployment (CI/CD) combined with Agile Methodology for faster, more predictable outcomes
Web Development
Our Enterprise app development encompasses both the Web and the Desktop platforms, delivering enhanced functionality for our clients.
Mobile Development
We focus on mobile-first thinking to create apps that deliver true value to users by acting as first-class citizens of the solution space.


ContinuousImprovements
Our belief in deploying advanced evolutions in digital era accelerates clients’ revenues by maintaining their constructive repute.
Data-Driven Marketing
We leverage our data analytics expertise to craft high impact marketing campaigns that take your digital presence to the next level via social media channels, search engine results and paid placements.
Quality Assurance
Our certified testers put the software through its paces not only in terms of its expected functionality but also in terms of its usability, resilience, speed, load, security and other aspects that have a direct impact on the overall perceived quality.
About Us
Our Vision Defines our Excellence. We are Committed to Digitize your Business. We are fully Focused on Designing, Developing, & Improving Intellectual Properties for your Business.
QUICK FACTS
- → Established in 2009
- → Headquartered in Hyderabad, India
- → Over 23 employees
- → 3 locations throughout the World
- → 90% of Service business originates from repeat customers